
Insights+: The US FDA New Drug Approvals in August 2022
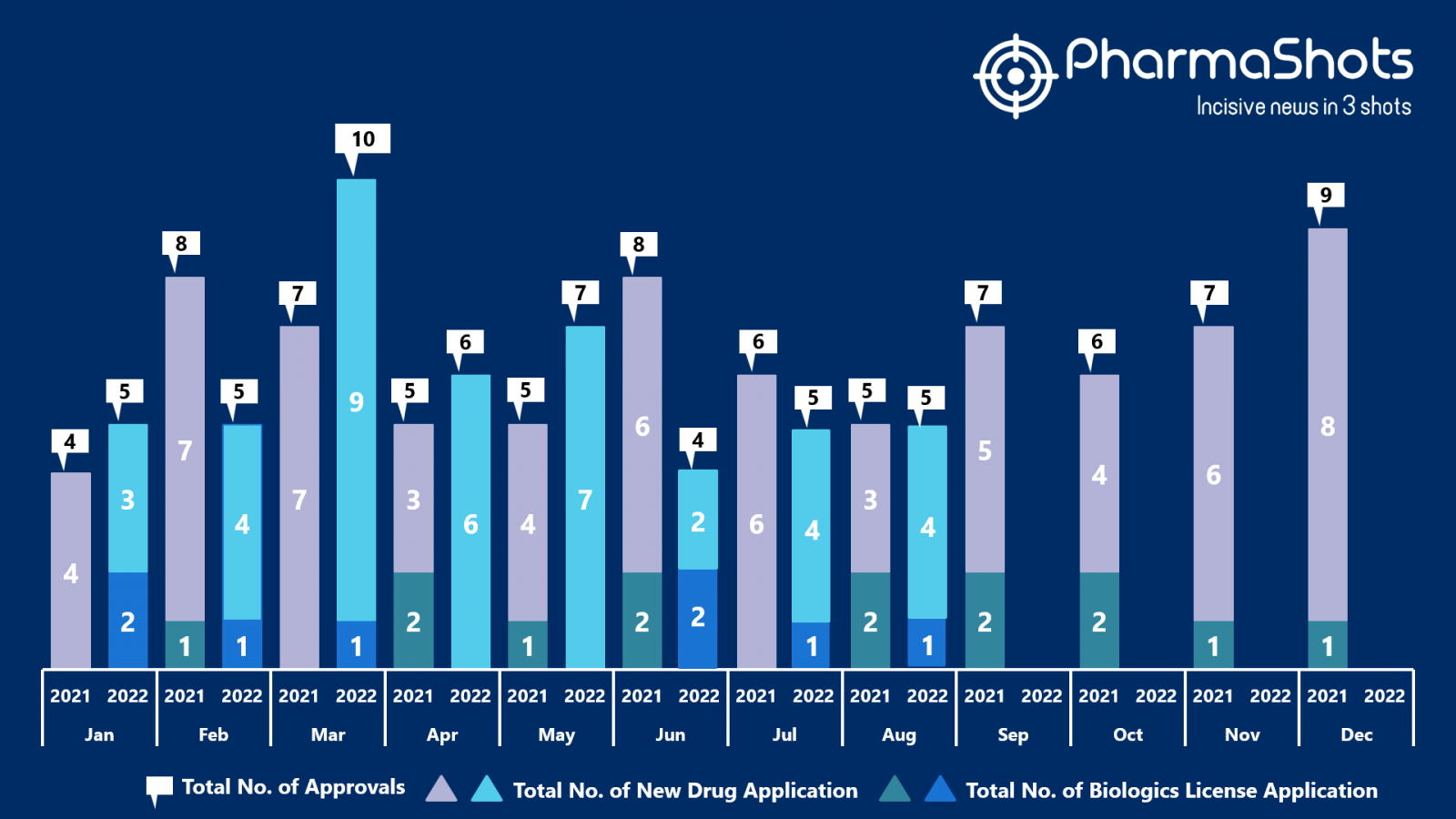
- The US FDA approved 4 NDAs and 1 BLA in August 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 47 novel products in 2022
- In August 2022, the major highlights drugs were Calquence's approval for blood cancer, Imbruvica (ibrutinib) for chronic graft versus host disease in pediatric patients, Xenpozyme (olipudase alfa-rpcp) for acid sphingomyelinase deficiency
- PharmaShots has compiled a list of a total of 5 new drugs approved by the US FDA in August 2022
Calquence
Active ingredient: acalabrutinib Approved: August 1, 2022
Company: AstraZeneca Disease: Blood Cancer
- The US FDA has approved Calquence as a new tablet formulation for all current indications incl. CLL, SLL & r/r MCL
- The approval was based on the (ELEVATE-PLUS) trials evaluating Calquence in 116 healthy patients which showed that acalabrutinib 100mg tablets were bioequivalent to the currently marketed acalabrutinib 100mg capsules indicating the same efficacy & safety profile with the same dosing strength. The tablet can be taken with gastric acid-reducing agents, incl. PPIs, antacids & H2-receptor antagonists & no new safety signals were reported
- Calquence is also approved as a capsule formulation for the same indications & as the tablet in the US and other countries globally
Axsome’s Auvelity Receives the US FDA’s Approval for the Treatment of Major Depressive Disorder
Auvelity
Active ingredient: dextromethorphan HBr -bupropion HCl Approved: August 19, 2022
Company: Axsome Therapeutics Disease: Blood Cancer
- The US FDA has approved Auvelity (dextromethorphan HBr -bupropion HCl) ER tablets for MDD. The therapy is expected to be available in the US in the Q4’22
- The approval was based on the P-III (GEMINI) trial to assess Auvelity in 327 patients which showed significant superiority in improving symptoms of depression over PBO, change in MADRS total score from baseline was significant at 1 & 2wk.
- In (ASCEND) study, the therapy was superior to bupropion sustained-release tablets (105mg, BID). The company launched the patient support program i.e., Auvelity On My Side which provides access to patients to use Auvelity & also offers patient support services, incl. the Auvelity On My Side savings card for eligible commercially-insured patients
Imbruvica
Active ingredient: ibrutinib Approved: August 25, 2022
Company: AbbVie Disease: Chronic Graft Versus Host Disease
- The US FDA has approved Imbruvica for cGVHD in pediatric patients aged ≥1yr. who required additional therapy after failure of ≥1 line of systemic therapy. The therapy is jointly developed & commercialized by Janssen & Pharmacyclics
- The approval was based on the P-I/II (iMAGINE) trial evaluating Imbruvica (in 47 pediatric & young adult patients aged 1yr. to ≤22yrs. with cGVHD which showed ORR (60%) @25wk. in patients aged 13yrs., m-DoR was 5.3mos. The safety was consistent with the established profile for Imbruvica, ARs consistent with those observed in cGVHD
- Imbruvica is a BTK inhibitor & was approved for a pediatric patient population. The therapy marks the first approved treatment option for children ≤12yrs. with cGVHD
Azurity’s Konvomep Receives the US FDA’s Approval for the Treatment of Active Benign Gastric Ulcer
Konvomep
Active ingredient: omeprazole and sodium bicarbonate Approved: August 31, 2022
Company: Azurity Pharmaceuticals Disease: Benign Gastric Ulcer
- The US FDA has approved Konvomep (omeprazole and sodium bicarbonate for oral suspension) for the treatment of active benign gastric. The therapy is also indicated for lowering the upper gastrointestinal bleeding risk in critically ill patients
- Konvomep is expected to be commercially available in Q1’23 & may give patients who have difficulty swallowing pills or capsules. Konvomep contains omeprazole (2mg), a proton pump inhibitor, and sodium bicarbonate per mL (84mg)
- The product is available in 90/150/300mL bottles. In July 2022, Azurity’s Zonisade has been approved in the US for partial seizures in adults and pediatric epilepsy patients aged ≥16yrs.
Xenpozyme
Active ingredient: olipudase alfa-rpcp Approved: August 31, 2022
Company: Sanofi Disease: Acid Sphingomyelinase Deficiency
- The approval was based on the (ASCEND) & (ASCEND-Peds) trials that evaluated Xenpozyme in 31 adult & 8 pediatric patients with ASMD (non-CNS manifestations) type A/B or type B for 52 & 64wks. Xenpozyme is expected to be available in the US in the coming wks.
- The results showed a mean reduction in spleen volume by (38.9%) from baseline to 52wk. over mean increase by 0.5% for patients in the PBO group in (ASCEND) trial & 46.7% in (ASCEND-Peds) trials; mean reduction in liver volume (26.5% vs 1.8%) & 38.1%; mean improvement in platelet count (18.3% vs 2.7%) & 37.6%
- In (ASCEND-Peds) trials, patients who performed the test at baseline had a mean relative improvement of 45.9% predicted DLco from baseline (48.5%) to 52wk. (70.9%) along with an improvement in lung function in both trials
Related Post: Insights+: The US FDA New Drug Approvals in July 2022
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














